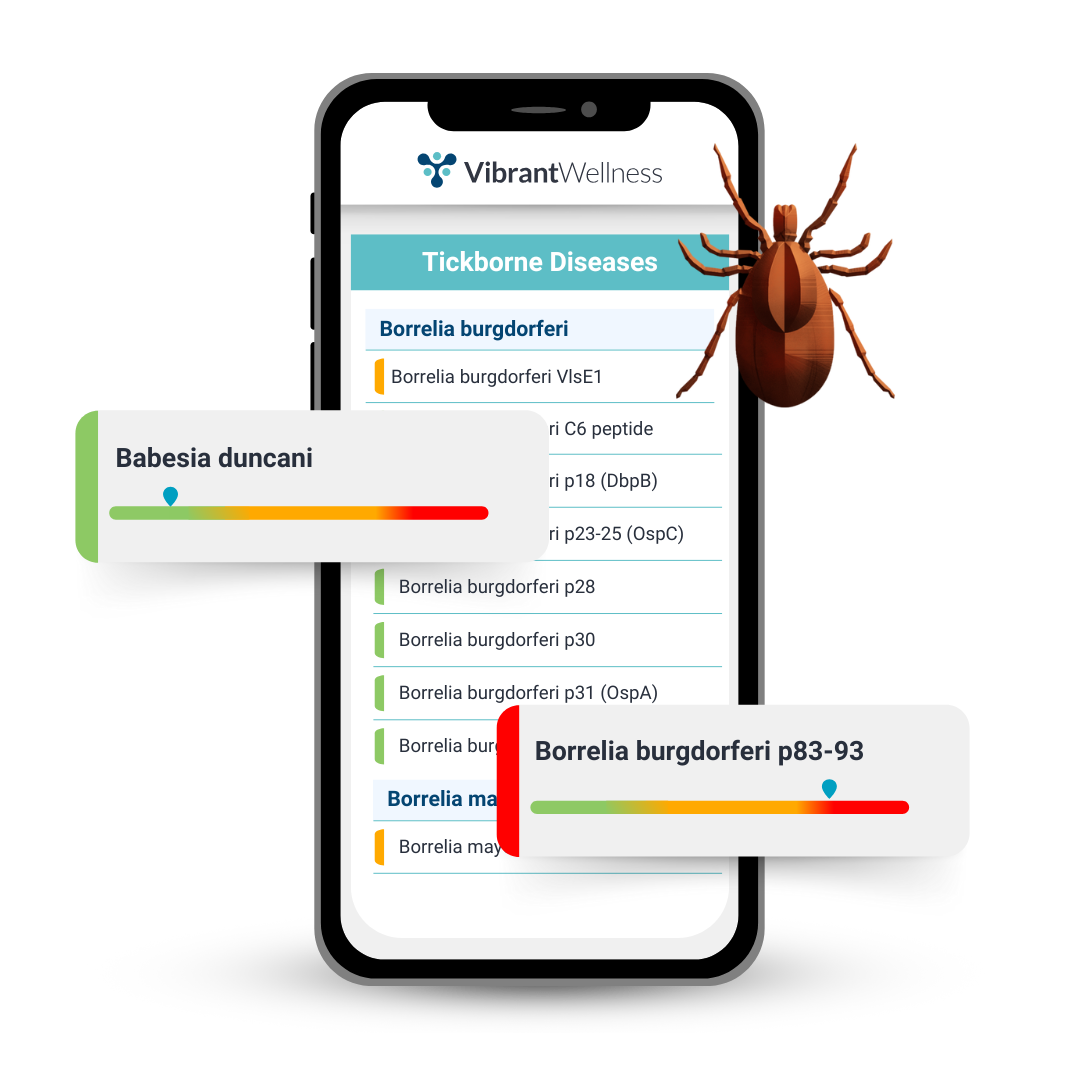
The Tickborne Diseases Panel is a comprehensive blood test that evaluates exposure to Lyme disease, tickborne co-infections, and related opportunistic infections. Utilizing advanced immunofluorescence, PCR, and microarray technology, this panel provides detailed insights into tickborne pathogen activity, supporting clinical evaluation and symptom management.
- Wide Range of Pathogens detects Lyme disease, tickborne relapsing fever, and co-infections like Babesia, Bartonella, Ehrlichia, and more, providing a broader range than traditional tests.
- Multiplex PCR & Immunoassay Technology enhances detection accuracy and sensitivity, even for co-infections that often go undiagnosed with standard tests.
- Comprehensive Tickborne Illness Insights supports early diagnosis of tick-related infections and autoimmune reactions, offering a complete understanding of underlying health issues.
- Guided Treatment Plans helps healthcare providers develop targeted intervention strategies for tickborne diseases and related health conditions.
Sample Report
Sample Type
NY State Available
Precision Testing For Personalized Solution
All of our laboratory tests are performed in a CLIA-certified and CAP-accredited facility in California


Why Order the Tickborne Diseases Panel

The Tickborne Diseases Panel tests for Lyme disease, tickborne relapsing fever, and a broad range of co-infections such as Babesia, Bartonella, and Ehrlichia, providing a more complete analysis than standard Lyme tests.
Tickborne illnesses can cause chronic, long-term health issues if left untreated. This test helps identify tickborne infections at an early stage, enabling timely intervention and treatment to prevent further complications.
If you suspect a tickborne illness, the Tickborne Diseases Panel provides clear, actionable results that help you understand your health status and guide your healthcare provider to develop a targeted treatment plan.
With detailed insights into tickborne infections, your healthcare provider can create a customized treatment plan to address co-infections and Lyme-related health issues, improving long-term wellness and preventing disease progression.
The Tickborne Diseases Panel provides a full evaluation of tickborne infections

About the Tickborne Diseases Panel
Borrelia burgdorferi Antigens:
- VlsE1
- C6 Peptide
- p18 (DbpB)
- p23-25 (OspC)
- p28
- p30
- p31 (OspA)
- p34 (OspB)
- p39 (BmpA)
- p41
- p45
- p58
- p66
- p83-93
- Borrelia burgdorferi B31 WCS
- Borrelia burgdorferi 297 WCS
Other Borrelia Species:
- Borrelia mayonii
- Borrelia afzelii (BmpA, DbpA, OspA, OspC, p100)
- Borrelia garinii (DbpA, OspC)
- Borrelia bavariensis (DbpA, p58, VlsE1)
- Borrelia spielmanii (DbpA, OspC)
- Borrelia hermsii
- Borrelia turicatae
- Borrelia miyamotoi
- Borrelia andersonii
- Borrelia maritima
- Borrelia californiensis
- Borrelia bissettiae
- Borrelia lusitaniae
- Borrelia valaisiana
- Borrelia yangtzensis
- Borrelia turcica
- Borrelia lonestari
Babesia Species:
- Babesia microti IRA
- Babesia microti p32
- Babesia microti p41
- Babesia microti WCS
- Babesia duncani
Anaplasma & Ehrlichia Species:
- Anaplasma phagocytophilum Msp5
- Anaplasma phagocytophilum Msp2 (p44)
- Anaplasma phagocytophilum OmpA
- Ehrlichia chaffeensis
Bartonella Species:
- Bartonella henselae 17 kDa
- Bartonella henselae 26 kDa
- Bartonella henselae SucB
- Bartonella elizabethae
- Bartonella vinsonii
- Bartonella quintana
Tickborne Viruses:
- Coxsackie Virus
- Powassan Virus
- Tickborne Encephalitis Virus
- West Nile Virus
Respiratory Bacterial Infections:
- Coxsackie Virus
- Powassan Virus
- Tickborne Encephalitis Virus
- West Nile Virus
Rickettsial Infections:
- Rickettsia typhi OmpB
- Rickettsia typhi Surface Antigen
- Rickettsia rickettsii
Herpesviruses & Other Opportunistic Infections:
- Cytomegalovirus EIA Antigen
- Cytomegalovirus GlyB
- Cytomegalovirus p150
- Cytomegalovirus p28
- Cytomegalovirus p52
- Cytomegalovirus p65
- Cytomegalovirus p38
- Epstein Barr Virus EA Antigen
- Epstein Barr Virus EBNA1
- Epstein Barr Virus VCA gp125
- Epstein Barr Virus p18
- Epstein Barr Virus p23
- Parvovirus B19 VLP VP2
- Parvovirus B19 VLP VP1/VP2 Co-Capsid
- Toxoplasma gondii Crude Extract
- Toxoplasma gondii MIC3
- Toxoplasma gondii p24
- Toxoplasma gondii p29
- Toxoplasma gondii p30
- HSV-1
- HSV-2
- HHV-6
- HHV-7
- Streptococcal A
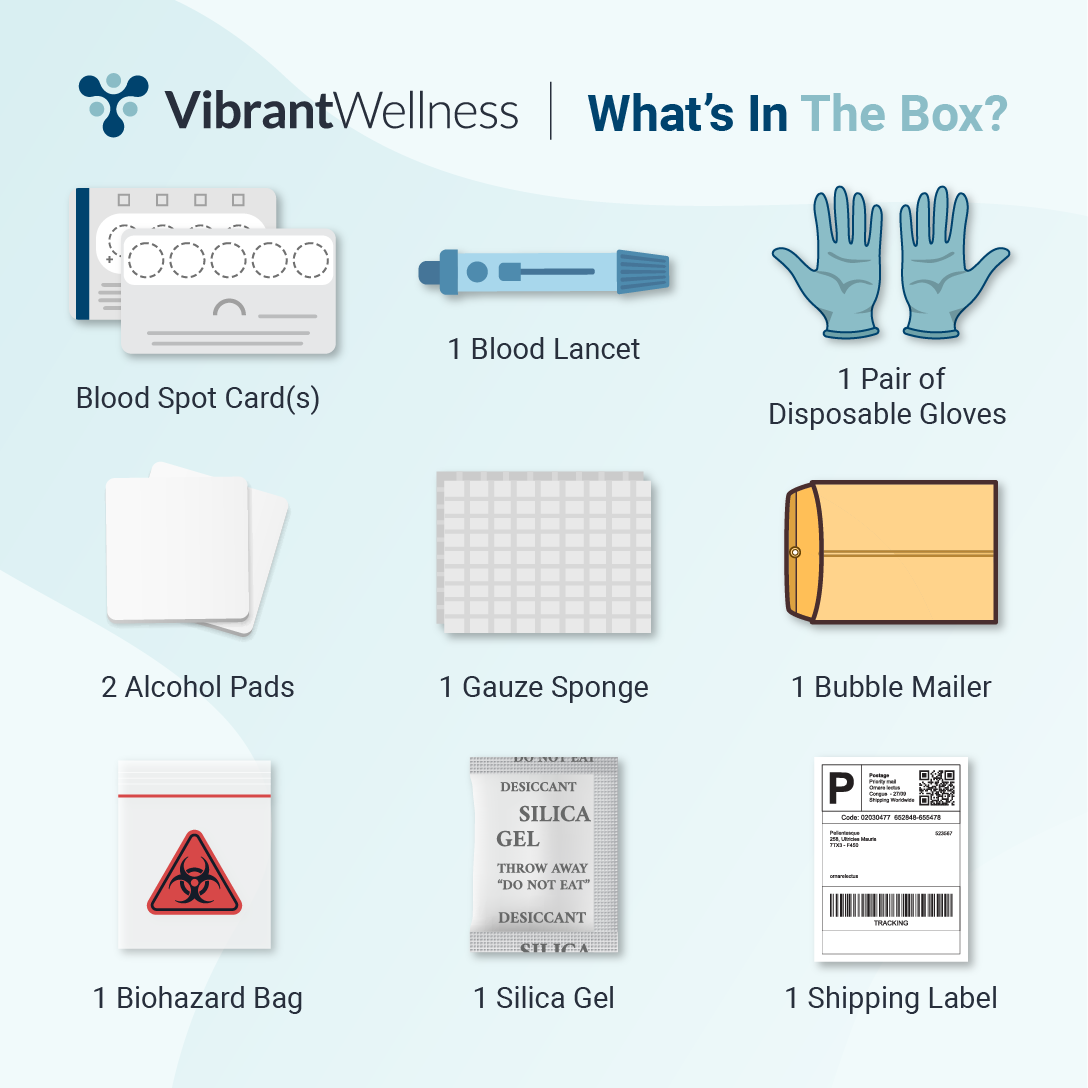
- No fasting required—maintain your normal diet.
- Hydration Considerations: No restrictions on fluid intake before collection.
- Dietary Considerations: No dietary restrictions before testing.
- Medication & Supplement Considerations: No medication or supplement restrictions; however, consult with your healthcare provider for personalized guidance.
- Ensure the sample is properly sealed and stored according to the provided instructions.
- Follow all collection procedures as directed to ensure accurate results.
Tickborne diseases, such as Lyme disease, Babesia, and Bartonella, can lead to chronic symptoms like fatigue, joint pain, and neurological issues if left undiagnosed.
Many tickborne infections are difficult to diagnose, often leading to misdiagnosis and prolonged symptoms that affect multiple systems in the body.
The Tickborne Complete 2.0 tests for Lyme disease and a broad range of co-infections, providing a complete analysis of tickborne pathogens and their impact on health.
Early detection of tickborne diseases allows for timely intervention, preventing long-term complications like chronic fatigue syndrome, arthritis, and neurodegenerative diseases.
By identifying tickborne infections early, individuals can begin targeted treatments to manage symptoms, reduce inflammation, and improve recovery outcomes.
This test provides a comprehensive view of tickborne diseases, helping healthcare providers create personalized treatment plans to address co-infections and Lyme-related health issues.
Borrelia burgdorferi Antigens:
- VlsE1
- C6 Peptide
- p18 (DbpB)
- p23-25 (OspC)
- p28
- p30
- p31 (OspA)
- p34 (OspB)
- p39 (BmpA)
- p41
- p45
- p58
- p66
- p83-93
- Borrelia burgdorferi B31 WCS
- Borrelia burgdorferi 297 WCS
Other Borrelia Species:
- Borrelia mayonii
- Borrelia afzelii (BmpA, DbpA, OspA, OspC, p100)
- Borrelia garinii (DbpA, OspC)
- Borrelia bavariensis (DbpA, p58, VlsE1)
- Borrelia spielmanii (DbpA, OspC)
- Borrelia hermsii
- Borrelia turicatae
- Borrelia miyamotoi
- Borrelia andersonii
- Borrelia maritima
- Borrelia californiensis
- Borrelia bissettiae
- Borrelia lusitaniae
- Borrelia valaisiana
- Borrelia yangtzensis
- Borrelia turcica
- Borrelia lonestari
Babesia Species:
- Babesia microti IRA
- Babesia microti p32
- Babesia microti p41
- Babesia microti WCS
- Babesia duncani
Anaplasma & Ehrlichia Species:
- Anaplasma phagocytophilum Msp5
- Anaplasma phagocytophilum Msp2 (p44)
- Anaplasma phagocytophilum OmpA
- Ehrlichia chaffeensis
Bartonella Species:
- Bartonella henselae 17 kDa
- Bartonella henselae 26 kDa
- Bartonella henselae SucB
- Bartonella elizabethae
- Bartonella vinsonii
- Bartonella quintana
Tickborne Viruses:
- Coxsackie Virus
- Powassan Virus
- Tickborne Encephalitis Virus
- West Nile Virus
Respiratory Bacterial Infections:
- Coxsackie Virus
- Powassan Virus
- Tickborne Encephalitis Virus
- West Nile Virus
Rickettsial Infections:
- Rickettsia typhi OmpB
- Rickettsia typhi Surface Antigen
- Rickettsia rickettsii
Herpesviruses & Other Opportunistic Infections:
- Cytomegalovirus EIA Antigen
- Cytomegalovirus GlyB
- Cytomegalovirus p150
- Cytomegalovirus p28
- Cytomegalovirus p52
- Cytomegalovirus p65
- Cytomegalovirus p38
- Epstein Barr Virus EA Antigen
- Epstein Barr Virus EBNA1
- Epstein Barr Virus VCA gp125
- Epstein Barr Virus p18
- Epstein Barr Virus p23
- Parvovirus B19 VLP VP2
- Parvovirus B19 VLP VP1/VP2 Co-Capsid
- Toxoplasma gondii Crude Extract
- Toxoplasma gondii MIC3
- Toxoplasma gondii p24
- Toxoplasma gondii p29
- Toxoplasma gondii p30
- HSV-1
- HSV-2
- HHV-6
- HHV-7
- Streptococcal A
- No fasting required—maintain your normal diet.
- Hydration Considerations: No restrictions on fluid intake before collection.
- Dietary Considerations: No dietary restrictions before testing.
- Medication & Supplement Considerations: No medication or supplement restrictions; however, consult with your healthcare provider for personalized guidance.
- Ensure the sample is properly sealed and stored according to the provided instructions.
- Follow all collection procedures as directed to ensure accurate results.
Tickborne diseases, such as Lyme disease, Babesia, and Bartonella, can lead to chronic symptoms like fatigue, joint pain, and neurological issues if left undiagnosed.
Many tickborne infections are difficult to diagnose, often leading to misdiagnosis and prolonged symptoms that affect multiple systems in the body.
The Tickborne Complete 2.0 tests for Lyme disease and a broad range of co-infections, providing a complete analysis of tickborne pathogens and their impact on health.
Early detection of tickborne diseases allows for timely intervention, preventing long-term complications like chronic fatigue syndrome, arthritis, and neurodegenerative diseases.
By identifying tickborne infections early, individuals can begin targeted treatments to manage symptoms, reduce inflammation, and improve recovery outcomes.
This test provides a comprehensive view of tickborne diseases, helping healthcare providers create personalized treatment plans to address co-infections and Lyme-related health issues.
Vibrant Advantage
The Tickborne Diseases Panel tests for Lyme disease and a broad range of co-infections, providing a comprehensive analysis of tickborne diseases that can cause chronic symptoms like fatigue, joint pain, and neurological issues.
The Tickborne Diseases Panel is backed by clinical validation, ensuring reliable results that support optimal tickborne disease management.
Compare Tickborne Diseases Panel with Autoimmune Zoomer Testing
The Tickborne Diseases Panel tests for Lyme disease and a broad range of tickborne co-infections such as Babesia, Bartonella, and Ehrlichia. Identifying these infections early can prevent chronic symptoms like fatigue, brain fog, and joint pain, which are often misdiagnosed.
The Autoimmune Zoomer complements the Tickborne Diseases Panel by identifying autoimmune responses that may be triggered by Lyme disease or co-infections. Chronic tickborne diseases can often lead to autoimmune reactions, exacerbating symptoms and complicating treatment.
Provides a comprehensive analysis of Lyme disease and its co-infections, offering critical data on the presence of tickborne pathogens that contribute to long-term health issues, especially when left untreated.
Identifies auto-antibodies and immune markers related to autoimmune conditions that may be activated by Lyme or co-infections, offering deeper insights into the immune response and providing more targeted management.
Measures antibodies and immune responses to Lyme disease and co-infections, including Babesia, Bartonella, and Ehrlichia, providing a clear view of tickborne pathogens in the body.
Measures a broad range of auto-antibodies and immune markers related to autoimmune diseases, giving healthcare providers a complete picture of immune dysfunction related to tickborne diseases.

The Tickborne Diseases Panel tests for Lyme disease and a broad range of tickborne co-infections such as Babesia, Bartonella, and Ehrlichia. Identifying these infections early can prevent chronic symptoms like fatigue, brain fog, and joint pain, which are often misdiagnosed.
Provides a comprehensive analysis of Lyme disease and its co-infections, offering critical data on the presence of tickborne pathogens that contribute to long-term health issues, especially when left untreated.
Measures antibodies and immune responses to Lyme disease and co-infections, including Babesia, Bartonella, and Ehrlichia, providing a clear view of tickborne pathogens in the body.
The Autoimmune Zoomer complements the Tickborne Diseases Panel by identifying autoimmune responses that may be triggered by Lyme disease or co-infections. Chronic tickborne diseases can often lead to autoimmune reactions, exacerbating symptoms and complicating treatment.
Identifies auto-antibodies and immune markers related to autoimmune conditions that may be activated by Lyme or co-infections, offering deeper insights into the immune response and providing more targeted management.
Measures a broad range of auto-antibodies and immune markers related to autoimmune diseases, giving healthcare providers a complete picture of immune dysfunction related to tickborne diseases.
FAQs for Tickborne Diseases Panel
How it works

Order Your Test
Your wellness provider orders your lab test

Activate Your Kit

Collect Your Sample

Ship Immediately

Receive Personalized Results
Featured Tests
- Lyme + TBRF Panel (Borrelia burgdorferi)
- Coinfections 1 Panel (Babesia microti, Babesia duncani, Bartonella spp., Anaplasma phagocytophilum, Ehrlichia chaffeensis)
- Coinfections 2 Panel (Rickettsia typhi, Chlamydophila pneumoniae, Mycoplasma pneumoniae)
-
Opportunistic Infections Panel (Toxoplasma gondii, Parvovirus B19)



.webp?width=869&height=878&name=1%20(3).webp)
