Perimenopause and Autoimmunity: Hormones, Gut Health, and Immune Disruption
Autoimmune conditions develop over time and exist on a spectrum of immune dysregulation. At one end, autoimmune activation occurs when the immune system mistakenly targets the body's own tissues. If left unchecked, this can progress to autoimmune disorders, where measurable tissue damage occurs. Eventually, this damage manifests as a diagnosed autoimmune disease, such as Hashimoto's thyroiditis, rheumatoid arthritis, celiac disease, or multiple sclerosis.
Unfortunately, autoimmune diseases disproportionately affect women, with studies indicating a four-fold increased risk compared to men, and 85% or more of multiple autoimmune disease patients are female.¹–²
Research suggests that the risk of autoimmune activation rises during major hormonal transitions, such as pregnancy and perimenopause.2 These hormone shifts influence immune function, gut health, and inflammation, creating a perfect storm for the autoimmune cascade.

At the root of autoimmunity are four key components:
- Genetics determines susceptibility but does not guarantee disease.
- Gut microbiome imbalance and leaky gut disrupt gut diversity and barrier integrity with hormonal shifts, increasing immune exposure to harmful antigens.
- Dysregulated immune function reduces immune tolerance, increasing reactivity.
- Environmental triggers push the immune system over the edge with stress, infections, toxins, and dietary factors.
The good news?
Autoimmune progression isn't inevitable. With early detection and targeted functional medicine interventions, it's possible to slow, halt, or reverse immune overactivation before lasting damage occurs. This article explores why autoimmunity surges in perimenopause, the underlying drivers, and practical strategies to restore immune tolerance, including key lab tests that can help you identify early immune dysregulation before making a formal diagnosis.
Table of Contents
|
Autoimmune Contributors: Why Perimenopause Creates the Perfect Storm |
|
|
Testing Pearls: Identifying Immune Vulnerabilities and Autoimmune Activation via Labs |
Autoimmune Contributors: Why Perimenopause Creates the Perfect Storm
1. Genetics Are Predisposed Terrain

Genetics plays a foundational role in autoimmune susceptibility, and it's common to see patterns of autoimmunity running in families. However, there is no single "autoimmune gene." Instead, research shows that autoimmune disorders have a complex genetic basis, with multiple genes contributing to disease risk.4
That said, genetic predisposition alone does not determine disease— the interplay between genetics, environmental exposures, and immune regulation dictates whether autoimmunity is triggered.5
This is why lifestyle medicine is essential for those with a family history of autoimmune disease and anyone looking to protect immune resilience. While genetics may increase susceptibility, autoimmune disease can develop in anyone if the immune system becomes overwhelmed by chronic stressors, gut dysfunction, inflammation, or environmental triggers.
2. The Gut-Immune Axis in Perimenopause
A healthy and balanced gut microbiome is critical for immune regulation, and the hormonal changes during perimenopause can significantly impact this delicate balance. Estrogen plays a key role in maintaining gut microbiome diversity, and as estrogen levels decline, microbiome diversity also decreases.6
This shift disrupts immune regulation, increases inflammation, and weakens gut barrier integrity, setting the stage for immune activation and autoimmunity.
Impact of Estrogen Decline on the Gut Microbiome
- Short-chain fatty acids (SCFAs) and immune regulation: A diverse, well-populated gut microbiota produces SCFAs, such as butyrate, crucial in promoting the differentiation and function of regulatory T cells (Tregs). Tregs help maintain immune tolerance by preventing overactive T cells from attacking the body's tissues.7 Therefore, as gut microbiome diversity declines, so does SCFA production, reducing the immune-regulating effects of Tregs and increasing the risk of immune dysregulation and inflammation.
- Pathogenic overgrowth and inflammation: An imbalance in the gut microbiota can lead to the overgrowth of pathogenic microbes, resulting in chronic inflammation and increased levels of endotoxins. This persistent inflammation provokes an immune system response—if left unresolved, it can lead to chronic immune activation, further driving immune dysregulation and autoimmune susceptibility.
Progesterone Decline, Relative Estrogen Excess, and Thyroid Function
Progesterone is the first hormone to decline during perimenopause. While estrogen also declines over time, its levels are highly erratic in the early stages of perimenopause, fluctuating between highs and lows.
In addition to natural estrogen fluctuations, total estrogenic load can be increased due to:
- Xenoestrogen exposure from plastics, personal care products, and pesticides.
- Increased adipose tissue that produces additional estrogen.
- Inefficient estrogen detoxification due to sluggish liver function, gut dysbiosis, or inadequate nutrients.
This hormonal imbalance—low progesterone alongside fluctuating or excessive estrogen—can increase the production of thyroid-binding globulin (TBG), which binds thyroid hormones and reduces the availability of free T3, the active form of thyroid hormone. Less free T3 available for cellular function compromises digestion, which in turn impacts immune regulation.⁸
How Thyroid Function Affects Digestion and Immunity
- Digestive hormone secretion: Suboptimal thyroid function reduces cholecystokinin (CCK), a hormone needed to stimulate pancreatic enzyme and bile release for proper fat digestion and absorption.
- Nutrient absorption: Impaired digestion hinders the absorption of fat-soluble vitamins. Vitamins A and D are especially critical for immune system function.
- Gastric acid production: Low thyroid function can lead to hypochlorhydria (low stomach acid), reducing the body's ability to absorb zinc, magnesium, vitamin C, and iron—all essential for immune and thyroid function.
These interconnected changes highlight the importance of gut health, hormonal balance, and thyroid function in supporting immune resilience during perimenopause.
Enhanced Intestinal Permeability and Its Link to Autoimmune Disease
Enhanced intestinal permeability (EIP), often called "leaky gut," has been implicated in the pathogenesis of various autoimmune diseases. Dr. Alessio Fasano's pioneering research has clearly established a connection between EIP and autoimmune activation.9
Intestinal Barrier Function and Immune Interaction
Under normal conditions, the intestinal barrier selectively permits the absorption of nutrients while restricting the passage of harmful substances. This regulation prevents luminal antigens, including toxin-producing and mucus-degrading pathogens, lipopolysaccharides (LPS), and pro-inflammatory mediators like interleukin-6 and tumor necrosis factor-alpha (TNF-α), from entering systemic circulation.
Hormonal Influences on Gut Barrier Integrity
Estrogen and progesterone play a critical role in maintaining intestinal barrier function. Their decline during perimenopause can compromise gut integrity, increasing the risk of EIP and immune dysregulation.
Estrogen's role in gut barrier protection:10- Protects mucus cells from oxidative injury, helping maintain the gut lining.
- Supports tight junctions of the epithelial barrier by increasing the expression of tight junction proteins, which regulate permeability and prevent harmful substances from entering systemic circulation.
- Upregulates occludin, a key tight junction protein, helping to reinforce the integrity of the intestinal lining.
- Has an inverse relationship with LPS—as progesterone declines, LPS levels may rise, leading to increased systemic inflammation and immune activation.
Additional Factors Promoting Enhanced Intestinal Permeability in Perimenopause
Several common scenarios during perimenopause may contribute to increased EIP, amplifying inflammation and immune dysregulation:
- Chronic stress: Life-stage stressors, such as career pressures, family dynamics, caregiving responsibilities, poor sleep, and physical discomfort (achy muscles and joints), can elevate cortisol levels. Increased cortisol has been associated with decreased expression of tight junction proteins, thereby enhancing intestinal permeability.11
- Alcohol consumption: Excessive alcohol can irritate and damage the cells lining the gut, leading to a breakdown of the protective barrier.13
- Vitamin D deficiency: Vitamin D influences the expression of structural proteins (claudins and occludins) integral to gut barrier tight junctions, making adequate levels essential for intestinal integrity.14
- Histamine excess:15
- Progesterone receptors on mast cells help regulate histamine release. As progesterone levels drop, this regulation weakens, leading to increased histamine, worsening gut permeability and contributing to inflammation.
- Elevated estrogen can further increase histamine by stimulating mast cells to release histamine and decreasing diamine oxidase (DAO), the enzyme responsible for breaking down ingested histamine.
- High estrogen states: High estrogen (and stress) states increase copper binding/retention via ceruloplasmin, which can lead to excess copper levels that block zinc absorption. Zinc is a critical nutrient for immune system function and gut barrier integrity.
- Use of oral contraceptives: Oral contraceptives, often prescribed to manage perimenopausal symptoms, may also contribute to intestinal permeability, further complicating immune regulation.16
Addressing these factors through lifestyle modifications, stress management, dietary interventions, and appropriate medical therapies is crucial for maintaining intestinal barrier integrity and reducing the risk of autoimmune activation during perimenopause.
3. Dysregulated Immune Function: The Hormonal-Immune Connection
A well-regulated immune system maintains tolerance, differentiating between harmful pathogens and benign substances, including self-tissues. The immune system's primary role is to tolerate, not attack. When dysregulation occurs, the immune system becomes overactive in an inappropriate and imbalanced way, similar to a cornered, wounded animal. This can manifest as:
- Elevated antibodies to benign substances, such as common foods, are seen in food sensitivity testing.
- Molecular mimicry, where the immune system mistakenly attacks self-tissue, confusing it with a pathogen due to structural similarities.
How Estrogen Regulates the Immune System
- Regulation of cytokines: Estrogen influences the production of cytokines, signaling molecules that mediate immune and inflammatory responses. By modulating cytokines, estrogen maintains an anti-inflammatory environment.17
- Immune response modulation: Estrogen interacts with estrogen receptor-beta (ER-β), which regulates key components of the innate immune response, such as the inflammasome. This interaction helps control inflammation and supports immune homeostasis.18
Impact of Estrogen Decline During Perimenopause
- Increased systemic inflammation: As estrogen declines, the body shifts toward a pro-inflammatory state, affecting various tissues and organs. This can contribute to joint pain, digestive issues, and increased susceptibility to inflammatory conditions.19
- Heightened immune activation: Chronic inflammation from low estrogen can overstimulate immune activity, reducing immune tolerance and increasing the risk of autoimmune responses.
4. What Triggers Push the System Over the Edge?

A single factor can trigger autoimmune disease. More often, the cumulative impact of multiple stressors overwhelms immune tolerance and activates autoimmunity. Perimenopause, a time of profound hormonal transition, already places the body in a more vulnerable state. When additional stressors accumulate, they can tip the balance toward autoimmune activation.
Common Triggers in Perimenopause:
- Major hormonal shifts: Life stages of significant hormonal fluctuations are known windows of increased autoimmune risk (i.e., pregnancy, perimenopause).
- Chronic stress and trauma: The ongoing demands of midlife—career pressures, caregiving, aging parents, and unresolved trauma—can lead to persistent cortisol elevation, which has been consistently linked to immune dysregulation and increased inflammation.20
- Dietary antigens and inflammatory foods: Gluten, dairy, and ultra-processed foods can drive inflammation and contribute to immune overactivation, particularly in susceptible individuals.
- Infections: Latent viruses (EBV, CMV, herpes), bacterial dysbiosis, and hidden infections can trigger autoimmune processes.
- Environmental toxins: Exposure to xenoestrogens, heavy metals, pesticides, and mold toxins places a persistent burden on the immune system, driving inflammation and immune dysregulation. These toxins can alter the gut microbiome, induce DNA damage, and trigger epigenetic changes, all of which can contribute to a misdirected immune response and increase the risk of autoimmune activation.21
- Sleep disruptions: 31-42% of perimenopausal women struggle with sleep issues, a major physiological stressor that weakens immune function and increases inflammation.22
Supporting hormonal balance, stress resilience, gut health, and toxin clearance can help reduce common triggers of autoimmune activation during perimenopause.
Testing Pearls: Identifying Immune Vulnerabilities and Autoimmune Activation via Labs
Lab testing can reveal underlying imbalances, nutrient deficiencies, toxic burdens, and early signs of autoimmunity before significant damage occurs.
Micronutrient Panel
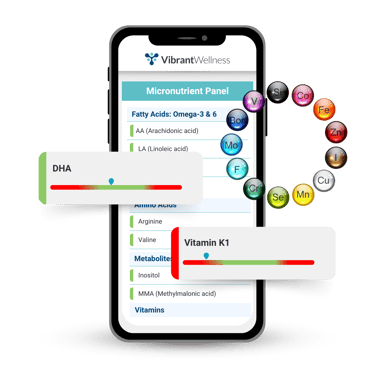 The Micronutrient Panel measures intra- and extracellular levels of vitamins, minerals, co-factors, amino acids, essential fatty acids, and antioxidants.
The Micronutrient Panel measures intra- and extracellular levels of vitamins, minerals, co-factors, amino acids, essential fatty acids, and antioxidants.
The immune system is one of the most nutrient-dependent systems in the body. This test helps identify key nutrient deficiencies that may impair immune function.
Autoimmune Zoomer
The Autoimmune Zoomer identifies specific autoimmune markers early and accurately by measuring autoantibodies and antigen interactions.
Provides detailed insights into immune activity, helping detect autoimmunity before significant tissue damage occurs.
 Total Tox Burden
Total Tox Burden
The Total Tox Burden is a urine test that evaluates total toxic burden by measuring mycotoxins, heavy metals, and environmental chemical excretion.
Chronic toxic exposures can contribute to immune dysregulation and autoimmune triggers.
Gut Zoomer
The Gut Zoomer is a comprehensive stool analysis that assesses over 170 bacterial species, gut pathogens, inflammatory markers, and digestive metabolites.
Gut dysbiosis and inflammation can be major drivers of autoimmune activation.
Food Sensitivity Panel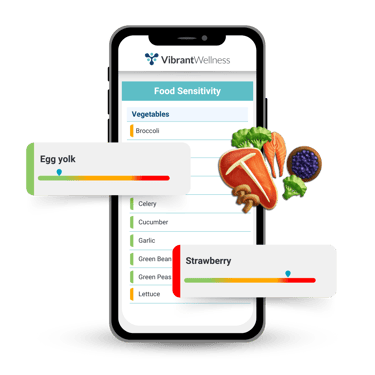
The Vibrant food sensitivity panels identify IgG and IgA antibody responses to common foods. This test helps detect delayed hypersensitivity reactions that often arise from intestinal permeability and contribute to immune hyperactivity.
Food Zoomers
The Vibrant Food Zoomers use peptide-based microarray technology to assess immune reactivity to food antigens at the peptide level. This high-resolution approach is especially useful for identifying immune triggers in patients with chronic inflammation or suspected autoimmunity.
Hormone Zoomer
The Hormone Zoomer is a urine panel that measures adrenal and sex hormones, including estrogen, progesterone, testosterone, DHEA-S, and cortisol, along with key metabolites.
It also evaluates endocrine disruptors and bone markers, offering insight into hormone patterns, detox capacity, and environmental exposures. The report includes supplement suggestions based on lab results and symptom data to guide targeted interventions during perimenopause.
Treatment and Clinical Applications: A Functional Roadmap
Targeted functional medicine interventions can restore immune balance, support gut integrity, and improve hormone regulation to prevent or slow autoimmune progression.
1. Restoring Gut Integrity and Immune Tolerance
- Identify and eliminate inflammatory foods, such as those containing gluten, dairy, processed foods, and potential triggers (nuts, seeds, nightshades) based on individual sensitivities.
- Support intestinal barrier function with L-glutamine, quercetin, vitamin D, zinc carnosine, and mucilaginous herbs (e.g., aloe vera, slippery elm, marshmallow, DGL licorice, okra, glucomannan).
- Add real, whole foods rich in phytonutrients, fiber, and digestive support where needed.
- Support microbiome diversity with:
- Prebiotic fiber and fermented foods (if tolerated).
- Probiotics that help regulate immune function: S. boulardii, L. plantarum,Ll. rhamnosus, Bifidobacterium species, and Akkermansia muciniphila.
- Soil-based organisms (SBOs) help restore immune tolerance, particularly in those with low akkermansia muciniphila.
Histamine-intolerant individuals should avoid L. casei, L. delbrueckii, L. helveticus, and Streptococcus species, which can exacerbate symptoms.
2. Immune Modulation Strategies
- Manage stress and nervous system regulation: Use heart rate variability (HRV) training, breathwork, and potentially adaptogens to regulate HPA axis function and cortisol balance.
- Optimize key nutrients for immune balance by ensuring adequate levels of:
- Vitamin D, zinc, selenium, and vitamin A to support immune function.
- Omega-3s and curcumin to modulate inflammation.
- Glutathione to support detoxification.
3. Hormonal Support for Immune Balance

- Bioidentical Progesterone may help regulate immune function, especially for progesterone-deficient individuals.
- Bioidentical Estrogen replacement therapy may sometimes be appropriate to preserve immune and metabolic balance.
Support estrogen metabolism and detoxification through:
- Lifestyle strategies that include increasing fiber intake, cruciferous vegetables, and liver support.
- Eliminating xenoestrogens in everyday items, such as plastics, pesticides, and synthetic hormones.
4. Prioritizing Sleep and Movement
- Sleep: Deep, restorative sleep is essential for immune repair, glymphatic system detoxification, and inflammation regulation.
- Movement: Individualized exercise should focus on:
- Lymphatic circulation and inflammation reduction.
- Joint mobility and pain reduction.
- Mood support and energy regulation.
Key Takeaways
Perimenopause is a critical window where autoimmune risk increases due to shifting hormones and immune dysregulation. Autoimmune susceptibility in perimenopause is driven by the interplay of genetics, gut health, immune regulation, and environmental triggers, but early functional testing can identify autoimmune activation before clinical diagnosis. A personalized, whole-body approach addressing gut health, immune modulation, hormone balance, sleep, and movement is essential for reducing autoimmune risk and supporting long-term immune resilience in midlife women.
About the Author
Alison Bame, RD, CFMP, is a Registered Dietitian, Certified Functional Medicine Practitioner, and Hormone Expert specializing in women's health, midlife weight management, and autoimmune conditions. With over 15 years of experience, she helps women over 40 navigate perimenopause, hormonal imbalances, and metabolic challenges using a root-cause, functional medicine approach. A passionate educator and advocate for proactive healthcare, Alison empowers women to take charge of their health through personalized nutrition, hormone balance, and gut-immune support. Learn more about her work at AlisonBame.com.
References:
- Kronzer VL, Bridges SL Jr, Davis JM III. Why women have more autoimmune diseases than men: An evolutionary perspective. Evol Appl. 2021;14(3):629-633. doi:10.1111/eva.13167. (https://pmc.ncbi.nlm.nih.gov/articles/PMC7980266/)
- Desai MK, Brinton RD. Autoimmune Disease in Women: Endocrine Transition and Risk Across the Lifespan. Front Endocrinol (Lausanne). 2019;10:265. doi:10.3389/fendo.2019.00265. (https://pmc.ncbi.nlm.nih.gov/articles/PMC6501433/)
- Farage MA, Miller KW, Maibach HI. Effects of menopause on autoimmune diseases. Expert Rev Obstet Gynecol. 2012;7(6):557-571. doi:10.1586/eog.12.54.
- Gregersen PK, Olsson LM. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol. 2009;27:363-391. doi:10.1146/annurev.immunol.021908.132653. (https://pmc.ncbi.nlm.nih.gov/articles/PMC2992886/)
- Mazzone R, Zwergel C, Artico M, et al. The emerging role of epigenetics in human autoimmune disorders. Clin Epigenetics. 2019;11(1):34. doi:10.1186/s13148-019-0632-2. (https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-019-0632-2)
- Peters BA, Santoro N, Kaplan RC, Qi Q. Spotlight on the Gut Microbiome in Menopause: Current Insights. Int J Womens Health. 2022;14:1059-1072. doi:10.2147/IJWH.S340491. (https://pmc.ncbi.nlm.nih.gov/articles/PMC9379122/)
- Luu M, Weigand K, Wedi F, et al. Microbiota-derived butyrate limits the autoimmune response by suppressing the differentiation of Th17 cells via the transcription factor RORγt. Immunity. 2021;54(1):111-123.e5. doi:10.1016/j.immuni.2020.12.001. (https://www.sciencedirect.com/science/article/pii/S2352396420302887)
- Sathi P, Kalyan S, Hitchcock CL, Pudek M, Prior JC. Progesterone therapy increases free thyroxine levels—data from a randomized placebo-controlled 12-week hot flush trial. Clin Endocrinol (Oxf). 2013;79(2):282-287. doi:10.1111/cen.12128. (pubmed.ncbi.nlm.nih.gov)
- Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:F1000 Faculty Rev-69. doi:10.12688/f1000research.20510.1. (pubmed.ncbi.nlm.nih.gov)
- Diebel ME, Diebel LN, Manke CW, Liberati DM. Estrogen modulates intestinal mucus physiochemical properties and protects against oxidant injury. J Trauma Acute Care Surg. 2015;78(1):94-99. doi:10.1097/TA.0000000000000499. (https://pubmed.ncbi.nlm.nih.gov/25539208/)
- Zhou Z, Bian C, Luo Z, et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci Rep. 2019;9(1):8367. doi:10.1038/s41598-019-44448-0. (https://www.nature.com/articles/s41598-019-44448-0)
- Bishehsari F, Magno E, Swanson G, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38(2):163-171. (pubmed.ncbi.nlm.nih.gov)
- Topalova-Dimitrova A, Dimitrov IV, Nikolov R. Lower vitamin D levels are associated with the pathogenesis of inflammatory bowel diseases. Medicine (Baltimore). 2023;102(41):e35505. doi:10.1097/MD.0000000000035505. (pmc.ncbi.nlm.nih.gov)
- Smolinska S, Winiarska E, Globinska A, Jutel M. Histamine: A Mediator of Intestinal Disorders—A Review. Metabolites. 2022;12(10):895. doi:10.3390/metabo12100895. (mdpi.com)
- Fleming CA, Costello M, O'Connor L, et al. The effect of sex, menstrual cycle phase, and oral contraceptive use on intestinal permeability. Physiol Behav. 2022;252:113804. doi:10.1016/j.physbeh.2022.113804. (https://www.sciencedirect.com/science/article/pii/S1043466622002009)
- Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine. 2015;72(2):121-129. doi:10.1016/j.cyto.2015.01.007. (pmc.ncbi.nlm.nih.gov)
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F. Estrogen receptor beta signaling alters cellular inflammasomes activity after global cerebral ischemia in reproductively senescence female rats. J Neurochem. 2015;132(6):777-787. doi:10.1111/jnc.13404. (https://onlinelibrary.wiley.com/doi/abs/10.1111/jnc.13404)
- Dall'Aglio L, Sánchez-Guerra M, Tarallo S, et al. Endogenous estradiol and inflammation biomarkers. Cancer Causes Control. 2020;31(8):767-780. doi:10.1007/s10552-020-01280-6.
- Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current directions in stress and human immune function. Curr Opin Psychol. 2015;5:13-17. doi:10.1016/j.copsyc.2015.03.007. (pubmed.ncbi.nlm.nih.gov)
- Kharrazian D. Exposure to environmental toxins and autoimmune conditions. Integr Med (Encinitas). 2021;20(2):20-24. PMID: 34377090; PMCID: PMC8325494. (https://pmc.ncbi.nlm.nih.gov/articles/PMC8325494/)
- Ciano C, King TS, Wright RR, Perlis M, Sawyer AM. Longitudinal study of insomnia symptoms among women during perimenopause. J Obstet Gynecol Neonatal Nurs. 2017;46(6):804-813. doi:10.1016/j.jogn.2017.07.011. (https://pubmed.ncbi.nlm.nih.gov/28886339/)
Regulatory Statement:
The general wellness test intended uses relate to sustaining or offering general improvement to functions associated with a general state of health while making reference to diseases or conditions. This test has been laboratory developed and its performance characteristics determined by Vibrant America LLC and Vibrant Genomics, a CLIA-certified and CAP-accredited laboratory performing the test. The lab tests referenced have not been cleared or approved by the U.S. Food and Drug Administration (FDA). Although FDA does not currently clear or approve laboratory-developed tests in the U.S., certification of the laboratory is required under CLIA to ensure the quality and validity of the test.

Alison Bame, RD, CFMP

Find a Provider- Take Control of Your Health
Connect with a provider to access Vibrant Wellness specialty tests and personalized insights that support your long-term health.
Related Articles
As a healthcare provider, understanding the link between gut health and cognitive function is crucial for supporting clients in optimizing their mental performa...

Thyroid diseases are the most common endocrine system disorders, affecting an estimated 5% of the US population, with Graves’ disease and Hashimoto’s thyroiditi...
.jpg)
Modern medicine has raised a critical question: How can we help people live better for longer? While lifespan measures the number of years lived, healthspan foc...







